Cell Junctions In Animal Cells
| Cell junction | |
|---|---|
| Details | |
| Identifiers | |
| Latin | junctiones cellulares |
| TH | H1.00.01.0.00012 |
| FMA | 67394 |
| Anatomical terminology [edit on Wikidata] | |
Jail cell junctions (or intercellular bridges [1]) are a class of cellular structures consisting of multiprotein complexes that provide contact or adhesion between neighboring cells or betwixt a cell and the extracellular matrix in animals. They also maintain the paracellular barrier of epithelia and control paracellular transport. Cell junctions are especially abundant in epithelial tissues. Combined with jail cell adhesion molecules and extracellular matrix, cell junctions help hold animal cells together.
Prison cell junctions are too especially important in enabling advice between neighboring cells via specialized protein complexes chosen communicating (gap) junctions. Cell junctions are likewise important in reducing stress placed upon cells.
In plants, like communication channels are known as plasmodesmata, and in fungi they are called septal pores.[2]
Types [edit]

Some examples of cell junctions
In vertebrates, there are iii major types of cell junction:
- Adherens junctions, desmosomes and hemidesmosomes (anchoring junctions)
- Gap junctions[3] (communicating junction)
- Tight junctions (occluding junctions)
Invertebrates have several other types of specific junctions, for case septate junctions or the C. elegans apical junction. In multicellular plants, the structural functions of cell junctions are instead provided for past cell walls. The analogues of communicative cell junctions in plants are chosen plasmodesmata.
Anchoring junctions [edit]
Cells within tissues and organs must be anchored to one another and fastened to components of the extracellular matrix. Cells have developed several types of junctional complexes to serve these functions, and in each case, anchoring proteins extend through the plasma membrane to link cytoskeletal proteins in one cell to cytoskeletal proteins in neighboring cells likewise as to proteins in the extracellular matrix.[4]
Three types of anchoring junctions are observed, and differ from one another in the cytoskeletal protein ballast as well every bit the transmembrane linker protein that extends through the membrane:
| Junction | Cytoskeletal ballast | Transmembrane linker | Ties cell to: |
|---|---|---|---|
| Desmosomes | Intermediate filaments | Cadherin | Other cells |
| Hemidesmosomes | Intermediate filaments | Integrins | EC matrix |
| Adherens junctions (Adhesion belt, Focal adhesion) | Actin filaments | Cadherin / Integrins | Other cells / EC matrix |
Anchoring-type junctions not simply concur cells together only provide tissues with structural cohesion. These junctions are most abundant in tissues that are subject to constant mechanical stress such as skin and centre.[4]
Desmosomes [edit]

This epitome shows a desmosome junction between cells of the epidermal layer of the pare.
Desmosomes, likewise termed equally maculae adherentes, can exist visualized as rivets through the plasma membrane of adjacent cells. Intermediate filaments composed of keratin or desmin are attached to membrane-associated zipper proteins that form a dense plaque on the cytoplasmic face of the membrane. Cadherin molecules course the actual anchor by attaching to the cytoplasmic plaque, extending through the membrane and bounden strongly to cadherins coming through the membrane of the next cell.[5]
Hemidesmosomes [edit]
Hemidesmosomes grade rivet-like links between cytoskeleton and extracellular matrix components such as the basal laminae that underlie epithelia. Like desmosomes, they necktie to intermediate filaments in the cytoplasm, merely in contrast to desmosomes, their transmembrane anchors are integrins rather than cadherins.[six]
Adherens junctions [edit]
Adherens junctions share the characteristic of anchoring cells through their cytoplasmic actin filaments. Similarly to desmosomes and hemidesmosomes, their transmembrane anchors are composed of cadherins in those that anchor to other cells and integrins (focal adhesion) in those that anchor to extracellular matrix. At that place is considerable morphologic diversity among adherens junctions. Those that tie cells to ane another are seen as isolated streaks or spots, or as bands that completely encircle the cell. The band-type of adherens junctions is associated with bundles of actin filaments that also encircle the cell just below the plasma membrane. Spot-like adherens junctions called focal adhesions help cells adhere to extracellular matrix. The cytoskeletal actin filaments that tie into adherens junctions are contractile proteins and in addition to providing an anchoring part, adherens junctions are idea to participate in folding and angle of epithelial jail cell sheets. Thinking of the bands of actin filaments as existence similar to 'drawstrings' allows one to envision how contraction of the bands inside a group of cells would distort the sheet into interesting patterns.[iv]
Communicating (gap) junctions [edit]
Communicating junctions, or gap junctions allow for direct chemic communication between adjacent cellular cytoplasm through diffusion without contact with the extracellular fluid.[7] This is possible due to six connexin proteins interacting to form a cylinder with a pore in the centre called a connexon.[8] The connexon complexes stretches across the cell membrane and when 2 next cell connexons interact, they course a complete gap junction channel.[seven] [eight] Connexon pores vary in size, polarity and therefore can exist specific depending on the connexin proteins that constitute each individual connexon.[7] [8] Whilst variation in gap junction channels do occur, their structure remains relatively standard, and this interaction ensures efficient communication without the escape of molecules or ions to the extracellular fluid.[eight]
Gap junctions play vital roles in the homo body,[9] including their role in the compatible contractile of the middle muscle.[9] They are also relevant in indicate transfers in the encephalon, and their absence shows a decreased cell density in the brain.[ten] Retinal and skin cells are also dependent on gap junctions in cell differentiation and proliferation.[nine] [10]
Tight junctions [edit]
Found in vertebrate epithelia, tight junctions act as barriers that regulate the movement of water and solutes between epithelial layers. Tight junctions are classified as a paracellular barrier which is defined as not having directional discrimination; however, motion of the solute is largely dependent upon size and charge. In that location is evidence to propose that the structures in which solutes pass through are somewhat like pores.
Physiological pH plays a part in the selectivity of solutes passing through tight junctions with most tight junctions being slightly selective for cations. Tight junctions present in different types of epithelia are selective for solutes of differing size, charge, and polarity.
Proteins [edit]
There take been approximately 40 proteins identified to be involved in tight junctions. These proteins can be classified into four major categories; scaffolding proteins, signalling proteins, regulation proteins, and transmembrane proteins.
Roles [edit]
- Scaffolding proteins – organise the transmembrane proteins, couple transmembrane proteins to other cytoplasmic proteins as well as to actin filaments.
- Signaling proteins – involved in junctions assembly, barrier regulation, and factor transcription.
- Regulation proteins – regulate membrane vesicle targeting.
- Transmembrane proteins – including junctional adhesion molecule, occludin, and claudin.
It is believed that claudin is the protein molecule responsible for the selective permeability between epithelial layers.
A iii-dimensional epitome is nonetheless nonetheless to exist achieved and as such specific information about the function of tight junctions is yet to be determined.
Tricellular junctions [edit]
Tricellular junctions seal epithelia at the corners of three cells. Due to the geometry of iii-cell vertices, the sealing of the cells at these sites requires a specific junctional organization, dissimilar from those in bicellular junctions. In vertebrates, components tricellular junctions are tricellulin and lipolysis-stimulated lipoprotein receptors. In invertebrates, the components are gliotactin and anakonda.[11]
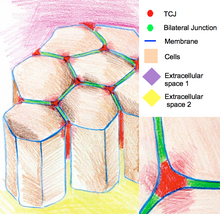
The cartoon of epithelium cells connected by tricellular junctions at the regions where three cells meet.
Tricellular junctions are also implicated in the regulation of cytoskeletal arrangement and cell divisions. In particular they ensure that cells divide co-ordinate to the Hertwig rule. In some Drosophila epithelia, during prison cell divisions tricellular junctions found concrete contact with spindle apparatus through astral microtubules. Tricellular junctions exert a pulling force on the spindle apparatus and serve as a geometrical clues to make up one's mind orientation of jail cell divisions.[12]
Jail cell junction molecules [edit]
The molecules responsible for creating cell junctions include diverse cell adhesion molecules. In that location are four primary types: selectins, cadherins, integrins, and the immunoglobulin superfamily.[13]
Selectins are cell adhesion molecules that play an important role in the initiation of inflammatory processes.[14] The functional chapters of selectin is limited to leukocyte collaborations with vascular endothelium. There are 3 types of selectins found in humans; L-selectin, P-selectin and E-selectin. L-selectin deals with lymphocytes, monocytes and neutrophils, P-selectin deals with platelets and endothelium and E-selectin deals only with endothelium. They take extracellular regions fabricated upward of an amino-last lectin domain, fastened to a carbohydrate ligand, growth factor-like domain, and short repeat units (numbered circles) that match the complementary binding poly peptide domains.[15]
Cadherins are calcium-dependent adhesion molecules. Cadherins are extremely of import in the process of morphogenesis – fetal evolution. Together with an alpha-beta catenin complex, the cadherin tin bind to the microfilaments of the cytoskeleton of the prison cell. This allows for homophilic prison cell–cell adhesion.[sixteen] The β-catenin–α-catenin linked complex at the adherens junctions allows for the formation of a dynamic link to the actin cytoskeleton.[17]
Integrins deed as adhesion receptors, transporting signals across the plasma membrane in multiple directions. These molecules are an invaluable role of cellular communication, as a single ligand can be used for many integrins. Unfortunately these molecules still have a long way to get in the ways of research.[18]
Immunoglobulin superfamily are a group of calcium independent proteins capable of homophilic and heterophilic adhesion. Homophilic adhesion involves the immunoglobulin-like domains on the jail cell surface binding to the immunoglobulin-like domains on an opposing cell'south surface while heterophilic adhesion refers to the binding of the immunoglobulin-like domains to integrins and carbohydrates instead.[xix]
Cell adhesion is a vital component of the body. Loss of this adhesion effects prison cell structure, cellular functioning and communication with other cells and the extracellular matrix and can lead to severe health issues and diseases.
References [edit]
- ^ Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson (2007). "Ch. 13: Box on morphology of squamous cell carcinoma". Robbins Bones Pathology (8th ed.). Philadelphia: Saunders. ISBN978-1-4160-2973-i.
- ^ Bloemendal, S; Kück, U (January 2013). "Cell-to-cell advice in plants, animals, and fungi: a comparative review". Die Naturwissenschaften. 100 (1): iii–19. Bibcode:2013NW....100....3B. doi:10.1007/s00114-012-0988-z. PMID 23128987. S2CID 11991859.
- ^ Andrew Fifty Harris; Darren Locke (2009). Connexins, A Guide. New York: Springer. p. 574. ISBN978-i-934115-46-six.
- ^ a b c Yan HH, Mruk DD, Lee WM, Cheng CY (2008). Cross-talk between tight and anchoring junctions-lesson from the testis . Advances in Experimental Medicine and Biology. Vol. 636. New York, NY : Springer-Verlag New York. pp. 234–54. doi:10.1007/978-0-387-09597-4_13. ISBN978-0-387-79990-ii. PMC4080640. PMID 19856171.
- ^ Lie PP, Cheng CY, Mruk DD (2011). The biological science of the desmosome-like junction a versatile anchoring junction and signal transducer in the seminiferous epithelium. International Review of Cell and Molecular Biology. Vol. 286. pp. 223–69. doi:x.1016/B978-0-12-385859-7.00005-seven. ISBN9780123858597. PMC4381909. PMID 21199783.
- ^ Gipson IK, Spurr-Michaud SJ, Tisdale AS (April 1988). "Hemidesmosomes and anchoring fibril collagen appear synchronously during development and wound healing". Developmental Biology. 126 (2): 253–62. doi:ten.1016/0012-1606(88)90136-4. PMID 3350210.
- ^ a b c Evans WH, Martin PE (2002). "Gap junctions: structure and function (Review)". Molecular Membrane Biology. nineteen (2): 121–36. doi:10.1080/09687680210139839. PMID 12126230. S2CID 20806078.
- ^ a b c d Lampe PD, Lau AF (July 2004). "The effects of connexin phosphorylation on gap junctional communication". International Journal of Biochemistry & Jail cell Biology. 36 (7): 1171–86. doi:10.1016/S1357-2725(03)00264-four. PMC2878204. PMID 15109565.
- ^ a b c "Abstracts: Proceedings of the International Gap Junction Conference. August 5–nine, 2007. Elsinore, Denmark". Cell Communication & Adhesion. 14 (vi): 275–346. 2007. doi:10.1080/15419060801891042. PMID 18392995.
- ^ a b Wei CJ, Xu X, Lo CW (2004). "Connexins and cell signaling in evolution and disease". Annual Review of Jail cell and Developmental Biology. 20: 811–38. doi:10.1146/annurev.cellbio.nineteen.111301.144309. PMID 15473861.
- ^ Byri Southward, Misra T, Syed ZA, Batz T, Shah J, Boril L, Glashauser J, Aegerter-Wilmsen T, Matzat T, Moussian B, Uv A, Luschnig South (2015). "The triple-echo poly peptide Anakonda controls epithelial tricellular junction germination in Drosophila". Developmental Prison cell. 33 (five): 535–48. doi:10.1016/j.devcel.2015.03.023. PMID 25982676.
- ^ Bosveld F, Markova O, Guirao B, Martin C, Wang Z, Pierre A, Balakireva Grand, Gaugue I, Ainslie A, Christophorou N, Lubensky DK, Minc N, Bellaïche Y (2016). "Epithelial tricellular junctions deed as interphase jail cell shape sensors to orient mitosis". Nature. 530 (7591): 496–eight. Bibcode:2016Natur.530..495B. doi:x.1038/nature16970. PMC5450930. PMID 26886796.
- ^ Lodish; et al. (2007). Molecular Cell Biology (6th ed.). West. H. Freeman and Visitor. p. 803. ISBN978-1429203142.
- ^ Tedder TF, Steeber DA, Chen A, Engel P (July 1995). "The selectins: vascular adhesion molecules". FASEB Journal. 9 (x): 866–73. doi:10.1096/fasebj.ix.10.7542213. PMID 7542213. S2CID 8315194.
- ^ Bevilacqua MP, Nelson RM (Feb 1993). "Selectins". Journal of Clinical Investigation. 91 (two): 379–87. doi:ten.1172/JCI116210. PMC287934. PMID 7679406.
- ^ Rowlands TM, Symonds JM, Farookhi R, Blaschuk OW (January 2000). "Cadherins: crucial regulators of structure and function in reproductive tissues". Reviews of Reproduction. five (1): 53–61. doi:10.1530/revreprod/v.1.53. PMID 10711736.
- ^ Brembeck FH, Rosário M, Birchmeier West (February 2006). "Balancing cell adhesion and Wnt signaling, the key office of β-catenin". Current Opinion in Genetics & Evolution. 16 (i): 51–9. doi:10.1016/j.gde.2005.12.007. PMID 16377174.
- ^ Hynes RO (September 2002). "Integrins: bidirectional, allosteric signaling machines". Cell. 110 (half dozen): 673–87. doi:10.1016/S0092-8674(02)00971-six. PMID 12297042. S2CID 30326350.
- ^ Wai Wong C, Dye DE, Coombe DR (2012). "The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis". International Journal of Cell Biology. 2012: 1–nine. doi:x.1155/2012/340296. PMC3261479. PMID 22272201.
External links [edit]
- Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002). "Cell Junctions". Molecular Biology of the Jail cell (4th ed.). New York: Garland Science. ISBN978-0-8153-3218-iii.
- Intercellular+Junctions at the US National Library of Medicine Medical Subject Headings (MeSH)
- Cell-Matrix+Junctions at the US National Library of Medicine Medical Field of study Headings (MeSH)
Cell Junctions In Animal Cells,
Source: https://en.wikipedia.org/wiki/Cell_junction
Posted by: hargravesyounter1970.blogspot.com


0 Response to "Cell Junctions In Animal Cells"
Post a Comment